Discovery lab for Reproductive and Child Health
Basic research focus
Our laboratory studies the formation and differentiation of human germline cells, the cells that ultimately give rise to eggs and sperm in adults. We use CRISPR/Cas9, next generation sequencing and pluripotent stem cells to develop new innovations in this research space. Our long-term research objective is to understand the fundamental principles of human germline cell formation during embryonic life towards the genesis of high quality gametes as adults and the birth of healthy children.
Regenerative medicine and translational research focus
More than ten thousand children under the age of fifteen in the United States will be diagnosed with cancer this year. More than 80% of these children will survive five years or more due to remarkable advances in cancer treatment. Treatments for childhood cancer includes combinations of surgery, radiation and chemotherapy, which can effectively kill cancer cells, but can also lead to serious side effects that cause permanent and irreversible tissue damage. One of the cells destroyed with cancer treatments are germline cells. Germline cells, which reside in the ovary and testis, are the only cell types in the body that enable us to have a baby of our own. One of the major regrets of childhood cancer survivors is their inability to have a family because they were unable to preserve/protect their fertility during cancer treatment. Using stem cells, our goal is to develop strategies to regenerate cells that are lost or damaged following cancer treatment so as to improve the quality of life for cancer survivors in the future. Our primary focus is to forge a path towards in vitro gametogenesis (IVG) to restore fertility. Discoveries from our lab could be a game changer for childhood cancer survivors who want to start a family as an adult, or for any individual where their germline has been destroyed through medical or environmental insults or as a result of combat.
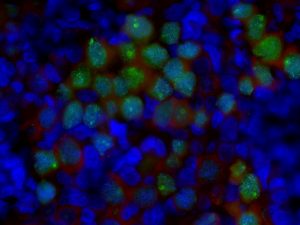
The Clark laboratory is well known for our pioneering work describing the cellular and molecular origins of the human germline. More recently, we have started to incorporate non-human primate studies to examine developmental stages in the germline that are impossible to access with human tissue. These projects are critical to the overall mission of our laboratory for two reasons. First, the study of human samples informs, stages and provides a measure of quality control for human germline cells differentiated in vitro from pluripotent stem cells. The second is that we are interested in understanding causes of human infertility and birth defects that originate during development. We want to ensure that the molecular pathways under investigation in laboratory models are found in the human germline and are, therefore, relevant to human reproductive and child health.
Shown are germ cells in the human fetal ovary (Image taken by Sofia Gkountela)
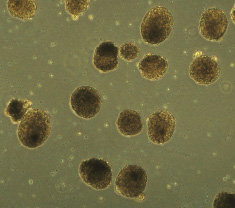
The Clark laboratory is committed to differentiating germline cells from pluripotent stem cells. We believe that this technology will serve as a model to understand causes of human infertility, identify genetic and epigenetic errors that originate in the germline that can cause birth defects in children, and as a potential therapeutic avenue to treat infertility in the future. We use three species of pluripotent stem cells in the laboratory. Mouse pluripotent stem cells, which can be used to not only study germline formation in a dish but can also be used in parallel with mouse modeling in vivo. Primate pluripotent stem cells, which can be used to determine functionality of germline cells formed in a dish by transplantation into clinically relevant non-human primate models of infertility. Human pluripotent stem cells, which are only used for in-dish modeling are currently used to teach us about the cellular and molecular origin of the human germline.
Shown are human embryoid bodies made from human stem cells (Image taken by Sofia Gkountela)
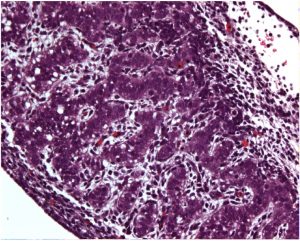
The Clark laboratory has established a new partnership with the laboratory of Kyle Orwig at the University of Pittsburgh and the laboratory of Marvin Meistrich and Gunapala Shetty at MD Anderson Cancer Center to develop a pre-clinical therapeutic approach to help boys overcome infertility following treatment for childhood cancers. We are excited about the future outcomes of this program project grant, as it will not only test whether non-human primate induced pluripotent stem cell-derived germline cells are transplantable, but enable unprecedented comparisons to cultured non-human primate spermatogonia. We are also excited to evaluate whether the testicular niche can be protected during radiation therapy, and whether this can improve reproductive outcomes in adult life.
Shown is a rhesus macaque embryonic testis (Image taken by Sofia Gkountela)
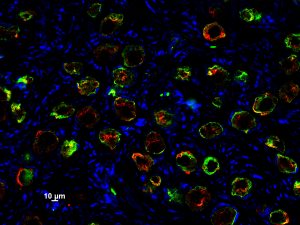
The Clark laboratory uses combinations of mouse genetics, mouse embryonic stem cells and analysis of human samples to identify the critical molecular pathways that ensure reproductive health and child health. We are particularly focused on the role of reversible changes to protein and DNA methylation in promoting germline quality. In this project, we have already published original work on the mechanisms responsible for promoting removal of cytosine methylation from the germline in order to erase epigenetic memory of earlier stages of development, including during gametogenesis in the parents. We are embarking on new work to evalute the mechanisms responsible for establishing methylation of DNA and proteins and their impact on germline quality.
Shown are germ cells in the embryonic human ovary (Image taken by Sofia Gkountela)
