The reproductive system is essential for health and wellness and is critical for the choice to start or build a family. The science of reproduction and reproductive wellness is complicated as many different organs and cell types are involved in a persons reproductive health and fertility. In order to develop solutions and cures for reproductive disease and dysfunction, particularly those that affect the ovary, members of the Clark Lab use single cell genomics, stem cell biology, organoids, gene editing and imaging technologies. Continue reading below for projects the Clark Lab are excited about. For more information about reproductive health and science on the UCLA campus, please see the UCLA Center for Reproductive Science, Health and Education
Project 1: The Ovary Project: Mapping Development and Differentiation of the Ovary to Improve Reproductive Health and Wellness
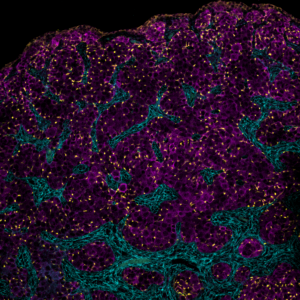
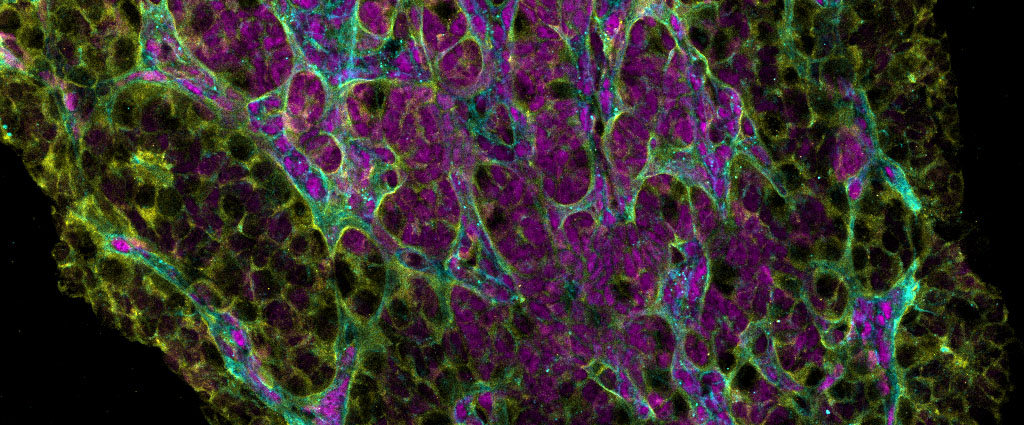
Image credit Sissy Wamaitha
The ovary is both a reproductive organ and an endocrine organ. These two ovarian functions are tightly linked as they both involve the functional units of the ovary called follicles. Follicles are composed of a single oocyte surrounded by one or more layers of granulosa cells. Problems with ovarian follicle establishment, or follicle cell biology will lead to diseases of the ovary including primary ovarian insufficiency, polycystic ovarian syndrome, and ovarian cancer. When follicle formation and function is abnormal, this causes endocrine disruptions that lead to precocious or delayed puberty, menstrual disorders, endometriosis or early onset menopause. Therefore, understanding the cell and molecular events involved in ovary development can lead to cures for ovarian disease and dysfunction which affect millions of women and girls around the world. To achieve this, our lab uses genomics, imaging and single cell analysis to map the cell lineages of the ovary. This basic knowledge can be used in projects to engineer ovaries from stem cells or to identify genes involved in ovarian disease and dysfunction. These projects are funded by the National Institute of Health, Institute for Child Health and Human Development. For our latest work on this project, please see our publications. For our single cell RNA-Seq work please visit our atlas to explore the data.
Project 2: Engineering Ovaries from Stem Cells
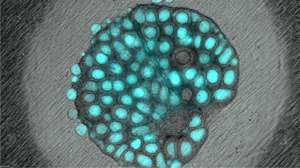
Image Credit Sinthia Kabir Mumu
Genome-wide association studies have identified a number of genes that may be involved in ovarian disease and dysfunction. A number of environmental agents, most notably environmental estrogens have also been shown to disrupt ovarian biology prior to birth, and in particular the establishment of the ovarian reserve. Testing the function of genes, or effects of environmental agents on ovarian biology are needed to improve public health interventions and prevent ovarian disease and dysfunction. To meet this need, we engineer models of the ovaries in the lab from stem cells. These models are called reconstituted ovaries (rOvaries) or reconstituted ovaroids (rOvaroids). We are currently using this technology as part of a screening platform for novel non-hormonal contraceptives in order to expand reproductive choice. This work is funded through the Ovarian Contraception Discovery Initiative, a project made possible through generous support from the Bill and Melinda Gates Foundation. For our latest work on this project, please see our publications. For the Ovarian Contraception Discovery Initiative, please visit the lead site at Northwestern Feinberg School of Medicine.
Project 3: Epigenetic Regulation of Germ Cell Development
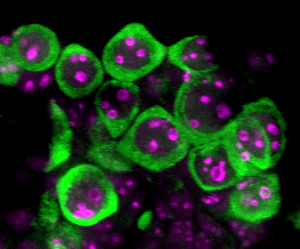
image credit Jon DiRusso
The mammalian germline undergoes successive waves of epigenetic remodeling to generate a gamete that is competent to support fertilization, embryo development and ultimately the birth of healthy offspring. Our lab studies the mechanisms of germline epigenetic remodeling in the earliest stages of germ cell development in the embryo. Epigenetic remodeling in the germline is a cumulative process. Therefore, if remodeling is incorrect from the outset, the next stages of germ cell development inherit the earlier epigenetic errors and these can be passed to offspring. To address epigenetic remodeling in early germ cells, we use genetically engineered models to study epigenetic regulators that act intrinsically in early germ cells to prepare them to respond to the niche and develop the capacity for initiating differentiation to undergo gametogenesis. Our work has shown that failed epigenetic remodeling in the early germline leads to problems with differentiation and infertility. Therefore epigenetic remodeling is a central requirement for human reproductive success and prevention of reproductive disease. Work on this project is funded by the National Institute of Health, National Institute for Child Health and Human Development. For our latest work on this project, please see our publications.
Project 4: Stem Cell Biology, Embryo Models and In vitro Gametogenesis
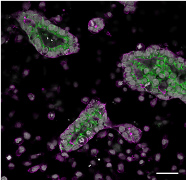
Image credit Auriana Arabpour
Pluripotent stem cells can differentiate into any cell type in the body including human germ cells. This makes human pluripotent stem cells an ideal research tool to understand genes, RNAs and proteins required for human germline development and reproduction. As human germ cells are amongst the earliest lineages to be specified in the first trimester human embryo, embryo modelling using human pluripotent stem cells can provide unique insights into the three-dimensional aspects, and tissue-tissue interactions involved in early human germline development. Diseases such as primary ovarian insufficiency have been associated with problems in primordial germ cell specification. Therefore understanding the events involved in germline establishment are critical to future studies aimed at identifying the genes involved in ovarian diseases such as primary ovarian insufficiency and precocious loss of endocrine support. This work is supported by the National Institute of Health, Institute for Child Health and Human Development. For social implications on the use of vitro gametogenesis for the purposes of reproduction, please see our colleagues work at the UCLA Institute of Society and Genetics https://socgen.ucla.edu.
Project 5: The Testis Project: Deciphering Cell Lineage Decisions During Embryonic Life
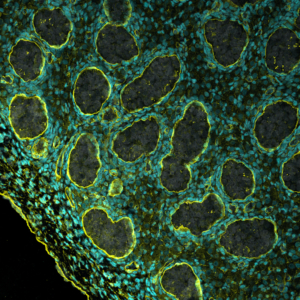
Image Credit Sissy Wamaitha
The testis is the major site in the body for testosterone production as well as tissue stem cells required for a persons fertility. The testis develops early in embryo development from multipotent precursors that have the capacity to differentiate into either an ovary or a testis depending on genetic sex. Our work in this area involves understanding how the germ cells of the testis develop and differentiate into tissue-specific stem cells called spermatogonia. In collaboration with our colleagues at the University of Pittsburgh and MD Anderson Cancer Center, we also demonstrated that germ cells made by in vitro gametogenesis are transplantable. Work on this project is funded by the National Institute of Health, National Institute for Child Health and Human Development. For our latest work on this project please see our publications.