Under construction
The role of Nkx2-5 and Notch in cardiogenesis
Cardiac progenitors give rise to diverse myocardial and non-myocardial lineages during cardiogenesis. Tight control of cellular specification, proliferation and migration is essential for normal heart formation. the proliferation of cardiomyocytes is regulated in a spatiotemporally distinct manner. Deep understanding of the mechanism in cardiogenesis sheds a light on the pathogenesis of congenital heart disease and the application of regenerative medicine.
Nkx2-5 is a key cardiac homeobox transcription factor that is expressed in broad range of cardiac sublineages from the early committed cardiac progenitors to the adult cardiomyocytes and required for the regulation of cardiac, vascular and hematopoietic lineages. Human heterozygous mutations of NKX2-5 are associated with a spectrum of CHD including atrial septal defect (ASD) and atrioventricular conduction block (AVB). However, the study of cell-intrinsic role of Nkx2-5 during the maturation of the cardiomyocytes at fetal stages has been hampered by early lethality of its straight knockout mice and hemodynamic alteration at later stages. In fact, hemodynamics by itself is known as an independent factor that regulates cardiomyocyte growth. Here we examined atrial-specific Nkx2-5 knockout by using Sln-Cre mice.
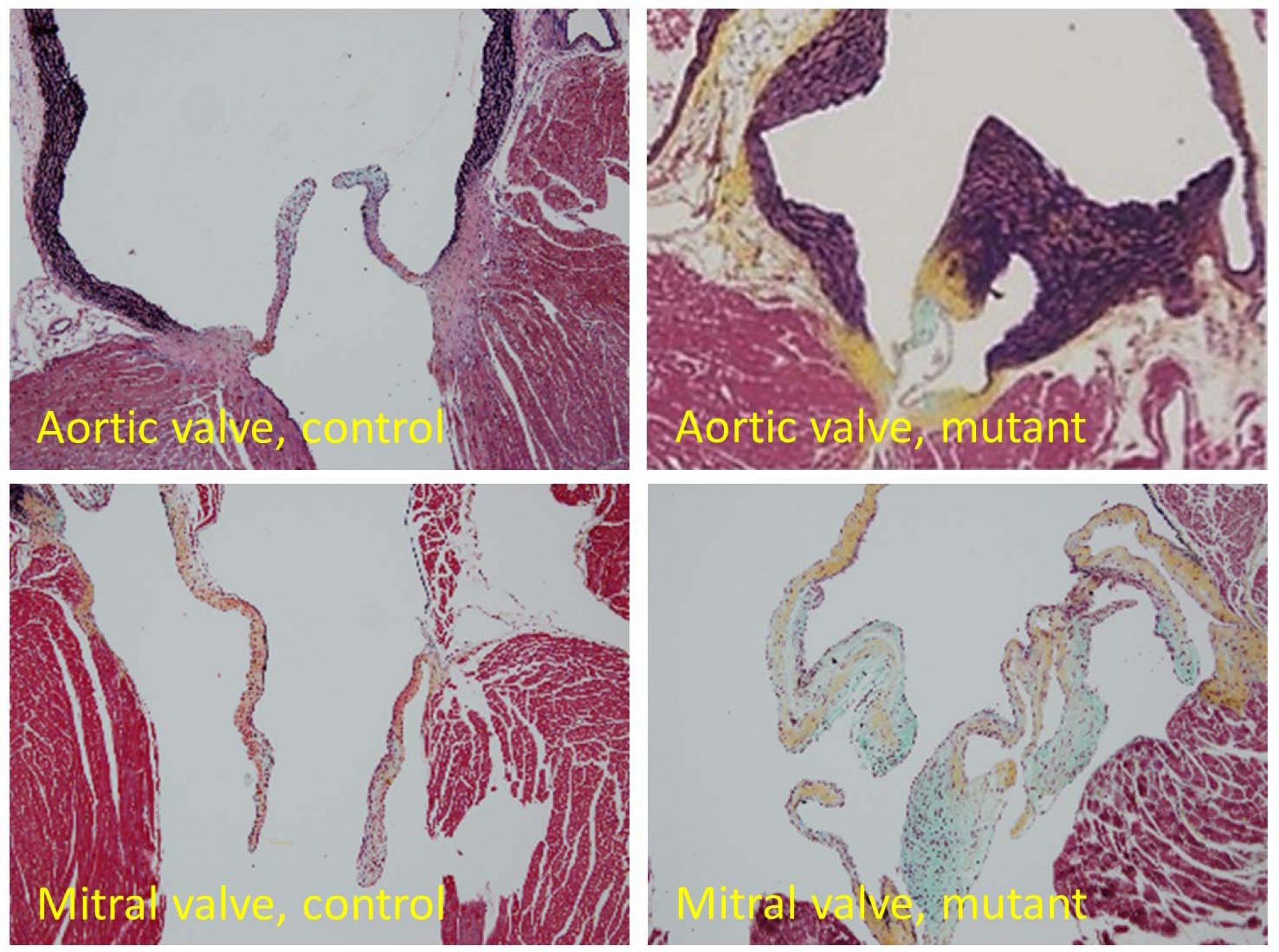
Atrial-specific Nkx2-5 mutants died shortly after birth with ASD, AVB and hyperplasia of working myocytes and conduction system including two nodes and internodal tracts. This phenotype is even more severe than ventricular specific knockouts, which survives until adult stages. Transcriptome analysis revealed that aberrant activation of Notch signaling underlies hyperproliferation of mutant cardiomyocytes. Consistently, forced activation of Notch signaling recapitulates hyperproliferation of working myocytes but not conduction system. These data suggest that Nkx2-5 suppresses proliferation of atrial working and conduction cardiomyocytes after chamber ballooning stages in coordination with Notch pathway. In fact, mutation of these genes leads to human congenital heart disease.
Nkx2-5 and Notch signaling are also linked in early cardiogenesis during the myogenesis, endocardial cushion formation and endocardial hematopoiesis.
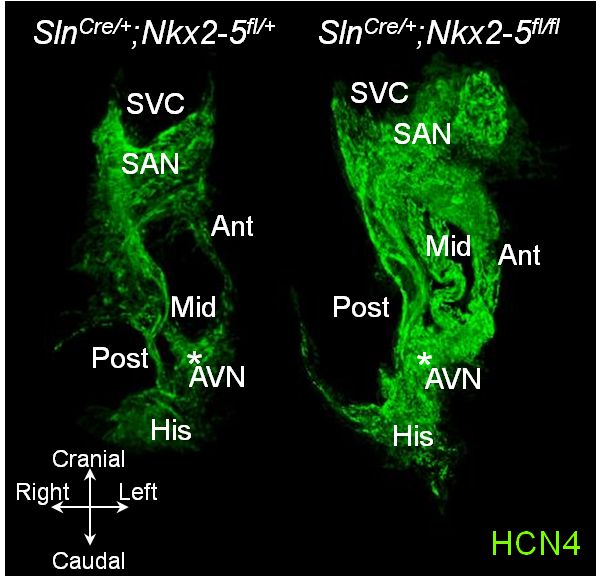
Hyperplastic conduction system in atrial-specific Nkx2-5 conditional knockout mice
Hyperplastic cardiac conduction system in Sln-Cre atrial-specific Nkx2-5 knockout mouse (right) and the control (left) in 3D reconstruction. Sinoatrial node (SAN), a
trioventricular node (AVN) and three internodal HCN4+ tracts between the two nodes (Ant, Mid, and Post) are markedly hyperplastic in atrial Nkx2-5-ablation. SVC, superior vene cava; His, His bundle. Video clips of the above figure follow. (Top Video is Control, Bottom Video is Mutant)
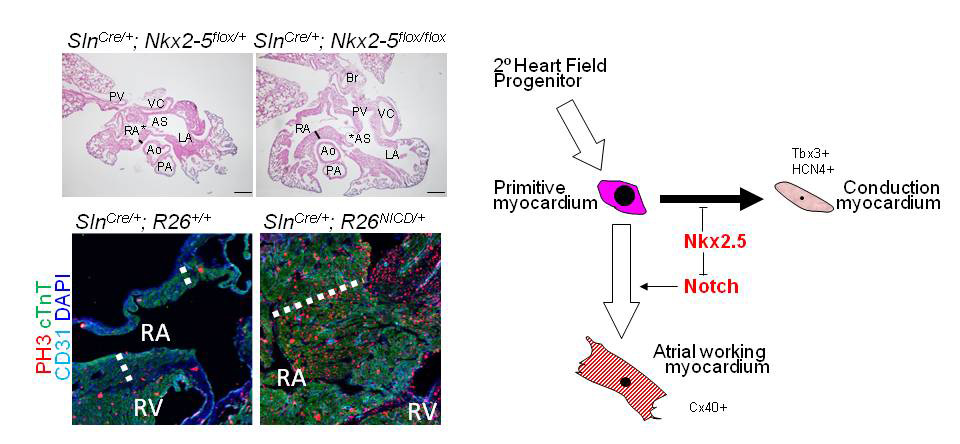
Nkx2-5-Notch axis in regulation of cardiomyocyte proliferation
Left: Atrial-Nkx2-5 ablation leads to hyperplastic atrial myocardium (thick line) and enlarged foramen ovale (asterisk). Activation of Notch signaling, which is upregulated in Nkx2-5-null atria in RNA-seq analysis, also leads to marked hyperplasia of atrial working myocytes (dotted line). Right: A schematic shows an interaction of Nkx2-5 and Notch signaling in regulation of cardiomyocyte proliferation in atria and conduction system. Ao, Aorta; AS, atrial septum; LA, left atria; PA, pulmonary artery; PV, pulmonary vein , RA, right atria; RV, right ventricle; VC, vena cava.