Members of our lab are engaged in a wide variety of projects that generally focus on mechanisms of plant function, the evolution of structural and physiological diversity, and implications for ecosystems under global change. We are very interested in applications of our research to conservation of species and ecosystems.
Several of the major research themes in our group are described below.
What determines plant responses to drought and their water use and how will drought influence species’ distributions in ecosystems worldwide?
Global change involves major shifts in patterns of rainfall and temperature such that the incidence and frequencies of droughts are increasing in ecosystems around the world. We are interested in clarifying the processes that unfold in droughted plants to better understand and predict the consequences for species under future climate scenarios. Additionally, we are interested in better quantifying plant water use, given the anticipated increasing scarcity, and the potential influence of contrasting species on watershed hydrology and water availability for other uses. Current collaborative projects include a focus on urban trees of Los Angeles, California native species, native versus invasive species of Hawaii, wheat cultivars, ecotypes of model species Arabidopsis thaliana, and comparing species globally with respect to their drought tolerance traits.
Selected recent publications:
 Anderegg WRL, Klein T, Bartlett M, Sack L, Pellegrini A, Choat B, Jansen S. 2016. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proceedings of the National Academy of Sciences USA, in press.
Anderegg WRL, Klein T, Bartlett M, Sack L, Pellegrini A, Choat B, Jansen S. 2016. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proceedings of the National Academy of Sciences USA, in press.
Bartlett MK, Zhang Y, Yang J, Kriedler N, Sun S-W, Lin L, Hu Y-H, Cao K-F, Sack L. 2016. Drought tolerance as a driver of tropical forest assembly: resolving spatial signatures for multiple processes. Ecology 97, 503-514. pdf Supporting Information
Bartlett M, Zhang Y, Kreidler N, Sun S, Ardy R, Cao K-F, Sack L. 2014. Global analysis of plasticity in turgor loss point, a key drought tolerance trait. Ecology Letters 12, 1580-1590. pdf Supporting Information
Cavaleri MA, Ostertag R, Cordell S, Sack L. 2014. Native trees show conservative water use relative to invasive: results from a removal experiment in a Hawaiian wet forest. Conservation Physiology 2, cou016. pdf
Maréchaux I, Bartlett M, Sack L, Baraloto C, Engel J, Joetzjer E, Chave J. 2015. Drought tolerance as predicted by leaf water potential at turgor loss point varies strongly across species within an Amazonian forest. Functional Ecology 29, 1268-1277. pdf Supporting Information
Hydraulic and stomatal control of leaf and plant water transport
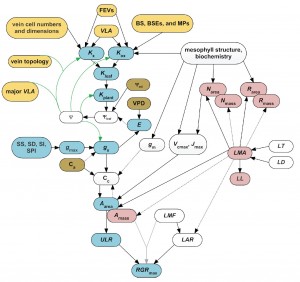
 The water transport system is a major determinant of plant productivity and adaptation to the environment. This system includes water movement through multiple organs and tissues within organs, and different cell types within tissues, a fascinating complexity with many features still in discovery phase. Our research focuses most strongly on the hydraulics of the leaf, a microhydrological system, in which water moves through veins and across living tissue before evaporating into airspaces and diffusing from the stomata. Projects in our lab examine the structural basis for leaf hydraulic and stomatal properties, and their dynamics and implications for plant (and ecosystem) performance, and, in collaboration with Tom Buckley of the University of Sydney and Herve Cochard of INRA, France, models for water transport within vein systems and living tissues, and for the influence of hydraulics on whole plant performance.
The water transport system is a major determinant of plant productivity and adaptation to the environment. This system includes water movement through multiple organs and tissues within organs, and different cell types within tissues, a fascinating complexity with many features still in discovery phase. Our research focuses most strongly on the hydraulics of the leaf, a microhydrological system, in which water moves through veins and across living tissue before evaporating into airspaces and diffusing from the stomata. Projects in our lab examine the structural basis for leaf hydraulic and stomatal properties, and their dynamics and implications for plant (and ecosystem) performance, and, in collaboration with Tom Buckley of the University of Sydney and Herve Cochard of INRA, France, models for water transport within vein systems and living tissues, and for the influence of hydraulics on whole plant performance.
Selected recent publications
 Sack L, Ball MC, Brodersen C, Davis SD, Des Marais DL, Donovan LA, Givnish TJ, Hacke UG, Huxman T, Jansen S, Jacobsen AL, Johnson D, Koch GW, Maurel C, McCulloh K, McDowell NG, McElrone A, Meinzer FC, Melcher PJ, North G, Pellegrini M, Pockman WT, Pratt RB, Sala A, Santiago LS, Savage JA, Scoffoni C, Sevanto S, Sperry J, Tyerman SD, Way D, Holbrook NM. 2016. Plant hydraulics as a hub integrating plant and ecosystem function. Plant, Cell & Environment, in press.
Sack L, Ball MC, Brodersen C, Davis SD, Des Marais DL, Donovan LA, Givnish TJ, Hacke UG, Huxman T, Jansen S, Jacobsen AL, Johnson D, Koch GW, Maurel C, McCulloh K, McDowell NG, McElrone A, Meinzer FC, Melcher PJ, North G, Pellegrini M, Pockman WT, Pratt RB, Sala A, Santiago LS, Savage JA, Scoffoni C, Sevanto S, Sperry J, Tyerman SD, Way D, Holbrook NM. 2016. Plant hydraulics as a hub integrating plant and ecosystem function. Plant, Cell & Environment, in press.
Sack L, Scoffoni C, Johnson DM, Buckley TN, Brodribb TJ. 2015. The anatomical determinants of leaf hydraulic function. in Functional and Ecological Xylem Anatomy, pp. 255-271. ed. UG Hacke. Springer, New York. pdf
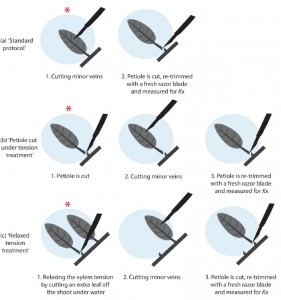 Scoffoni C, Sack L. 2015. Are leaves “freewheelin'”? Testing for a Wheeler-type effect in leaf xylem hydraulic decline. Plant, Cell & Environment 38, 534-543. pdf Supporting Information
Scoffoni C, Sack L. 2015. Are leaves “freewheelin'”? Testing for a Wheeler-type effect in leaf xylem hydraulic decline. Plant, Cell & Environment 38, 534-543. pdf Supporting Information
Buckley TN, John GP, Scoffoni C, Sack L. 2015. How does leaf anatomy influence water transport outside the xylem? Plant Physiology 168, 1616-1635. pdf
Scoffoni C, Vuong C, Diep S, Cochard H, Sack L. 2014. Leaf shrinkage with dehydration: coordination with hydraulic vulnerability and drought tolerance. Plant Physiology 164, 1772-1788. pdf Supplemental Data
Locke AM, Sack L, Bernacchi CJ, Ort DR. 2013. Soybean leaf hydraulic conductance does not acclimate to growth at elevated [CO2] or temperature in growth chambers or in the field. Annals of Botany 112: 911-918. pdf Supplementary Data
Evolution and functional consequences of diversity in leaf designs
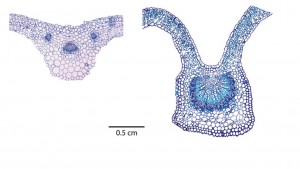
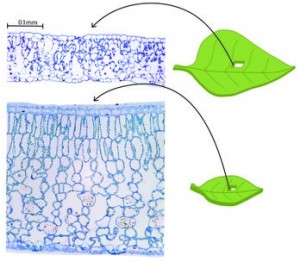
 Leaves vary tremendously in size, shape, anatomy, venation, surface features, gas exchange and drought tolerance. Often, great variation occurs even among closely related species (as in different maple species or cycad species; see right, above and below respectively). Our research is determining the evolutionary history and functional consequences of leaf diversification. We have focused on all major plant groups (lycophytes, ferns, conifers and angiosperms), with recent and current collaborative projects focus on diverse lineages and adaptive radiations, including Viburnum (Adoxaceae), lobeliads (Campanulaceae), Euphorbias (Euphorbiaceae), Ceanothus (Rhamnaceae), Scaevola (Goodeniaceae), Ficus (Moraceae), ferns, cycads and grasses. We are also interested in large scale trait analyses across large numbers of lineages.
Leaves vary tremendously in size, shape, anatomy, venation, surface features, gas exchange and drought tolerance. Often, great variation occurs even among closely related species (as in different maple species or cycad species; see right, above and below respectively). Our research is determining the evolutionary history and functional consequences of leaf diversification. We have focused on all major plant groups (lycophytes, ferns, conifers and angiosperms), with recent and current collaborative projects focus on diverse lineages and adaptive radiations, including Viburnum (Adoxaceae), lobeliads (Campanulaceae), Euphorbias (Euphorbiaceae), Ceanothus (Rhamnaceae), Scaevola (Goodeniaceae), Ficus (Moraceae), ferns, cycads and grasses. We are also interested in large scale trait analyses across large numbers of lineages.
Selected recent publications:
 Chatelet DS, Clement WL, Sack L, Donoghue MJ, Edwards EJ. 2013. The evolution of photosynthetic anatomy in Viburnum (Adoxaceae). International Journal of Plant Sciences 174, 1277-1291. pdf Supplements
Chatelet DS, Clement WL, Sack L, Donoghue MJ, Edwards EJ. 2013. The evolution of photosynthetic anatomy in Viburnum (Adoxaceae). International Journal of Plant Sciences 174, 1277-1291. pdf Supplements
Creese C, Oberbauer S, Rundel P, Sack L. 2014. Are fern stomatal responses to different stimuli coordinated? Testing responses to light, vapor pressure deficit and CO2 for diverse species grown under contrasting irradiances. New Phytologist 204, 92-104. pdf Supporting Information
Edwards E, Chatelet D, Garrison L, Sack L, Donoghue MJ. 2014. Leaf lifespan and the leaf economic spectrum in the context of whole plant architecture. Journal of Ecology 102, 328-336. pdf
Hao G, Wang A-Y, Sack L, Goldstein G, Cao K-F. 2013. Is hemiepiphytism an adaptation to high irradiance? Testing seedling responses to light levels and drought in hemiepiphytic and non-hemiepiphytic Ficus. Physiologia Plantarum 148, 74-86. pdf
Scoffoni C, Kunkle J, Pasquet-Kok J, Vuong C, Patel A, Montgomery R, Givnish TJ, Sack L. 2015. Light-induced plasticity in leaf hydraulics, venation, anatomy and gas exchange in ecologically diverse Hawaiian lobeliads. New Phytologist 207, 43-58. pdf Supporting Information
Zhang Y-J, Cao K-F, Sack L, Li N, Wei X-M, Goldstein G. 2015. Extending the generality of leaf economic design principles in the cycads, an ancient lineage. New Phytologist 206, 817-829. pdf Supporting Information
How does variation in genetic architecture and developmental processes determine plant structure, function and performance?
 We have recently taken on a focus on determining the underlying genetic basis for variation in anatomy and physiology across mutants and ecotypes of model species Arabidopsis thaliana (project funded by the National Science Foundation, in collaboration with Matteo Pellegrini, UCLA Dept of Molecular Cell and Developmental Biology) and populations of California live oaks (project funded by the National Science Foundation, in collaboration with Victoria Sork, UCLA Dept of Ecology and Evolutionary Biology). In addition, we are elucidating the consequences of the often constrained plant developmental algorithms for shaping plant structures and how they diversify across environments today and in the deep past.
We have recently taken on a focus on determining the underlying genetic basis for variation in anatomy and physiology across mutants and ecotypes of model species Arabidopsis thaliana (project funded by the National Science Foundation, in collaboration with Matteo Pellegrini, UCLA Dept of Molecular Cell and Developmental Biology) and populations of California live oaks (project funded by the National Science Foundation, in collaboration with Victoria Sork, UCLA Dept of Ecology and Evolutionary Biology). In addition, we are elucidating the consequences of the often constrained plant developmental algorithms for shaping plant structures and how they diversify across environments today and in the deep past.
Selected recent publications:
Caringella MA, Bongers FJ, Sack L. 2015. Leaf hydraulic conductance varies with vein anatomy across Arabidopsis thaliana wild-type and leaf vein mutants. Plant, Cell & Environment 38, 2735-2746. pdf Supporting Information
John GP, Scoffoni C, Sack L. 2013. Allometry of cells and tissues within leaves. American Journal of Botany 100: 1936-1948. pdf Supplementary Data
Sack L, Scoffoni C, McKown AD, Frole K, Rawls M, Havran JC, Tran H, Tran T. 2012. Developmentally based scaling of leaf venation architecture explains global ecological patterns. Nature Communications 3: 837. pdf Supporting Information
How do diverse tissue and organ structures and physiological processes scale up to plant and ecosystem function and their responses to climate change?
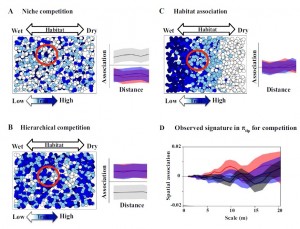
We are physiologists and ecologists (or ecophysiologists), and generally fascinated with the diversity of plant traits and their implications for species’ specializations in habitat, response to environment and interactions with other organisms. Current projects include the diversity and function of leaf structure, including the venation, stomata and hairs; leafand wood economics; predicting plant growth and habitat adaptation from multiple traits; understanding global diversity and environmental correlations of plant traits; ecological patterning of seedlings and trees; modeling ecosystem productivity on the basis of plant traits across forests worldwide; applications of trait relationships to fossils to clarify plant ecology and climate in the deep past. Current study systems include California native species, Hawaiian native forests (in collaboration with the Hawaii Permanent Plot Network; HIPPNET), and the Los Angeles urban forest; recent collaborative work has focused on Australian, Bolivian and Chinese rain forests.
Selected recent publications:
 Inman-Narahari F, Ostertag R, Hubbell S, Giardina C, Cordell S, Sack L. 2016. Density-dependent seedling mortality varies with light availability and species abundance in wet and dry Hawaiian forests. Journal of Ecology, in press.
Inman-Narahari F, Ostertag R, Hubbell S, Giardina C, Cordell S, Sack L. 2016. Density-dependent seedling mortality varies with light availability and species abundance in wet and dry Hawaiian forests. Journal of Ecology, in press.
Merkhofer L, Wilf P, Haas MT, Kooyman RM, Sack L, Scoffoni C, Cuneo NR. 2015. Resolving Australian analogs for an Eocene Patagonian paleorainforest using leaf size and floristics. American Journal of Botany 102, 1160-1173. pdf Supporting Information
Pivovaroff A, Sharifi R, Scoffoni C, Sack L, Rundel P. 2014. Making the best of the worst of times: traits underlying the combined shade and drought tolerance of Ruscus aculeatus and R. microglossum (Asparagaceae). Functional Plant Biology 41, 11-24. pdf
Mendez-Alonzo R, Ewers F, Sack L. 2013. Ecological variation in leaf biomechanics and its scaling with tissue structure across three mediterranean-climate plant communities. Functional Ecology 27, 544-554. pdf Supporting Information
Sack L, Scoffoni C. 2013. Tansley Review. Leaf venation: structure, function, development, evolution, ecology and applications in past, present and future. New Phytologist 198, 983-1000. pdf Supporting Information
Sack L, Scoffoni C, John GP, Poorter H, Mason CM, Mendez-Alonzo R, Donovan LA. 2013. How do leaf veins influence the worldwide leaf economic spectrum? Review and synthesis. Journal of Experimental Botany 64: 4053-4080. pdf Supplementary Data
Global analyses of physiology of species and ecosystems
Projects in the lab focus on applying approaches from physiology and ecology to characterizing plant species and ecosystems worldwide and predicting their responses to climate change. Several major projects focus on the native wet and dry forests of Hawaii (in collaboration with the Hawaii Permanent Plot Network; HIPPNET), as these are excellent model systems as well as critical systems to understand and preserve. We also conduct meta-analyses of global databases and collaborate with the network of researchers studying forests worldwide.
Selected recent publications:
 Anderson-Teixeira KJ, Davies SJ, Bennett AC, Gonzalez-Akrea EB, Muller-Landau HC, Wright SJ, Abu Salim K, Almeyda Zambrano AM, Alonso A, Baltzer JL, Basset Y, Bourg NA, Broadbent EN, Brockelman WY Bunyavejchewin S, Burslem DFRP, Butt N, Cao M, Cardenas D, Chuyong GB, Clay K, Cordell S, Dattaraja HS, Deng X, Detto M, Du X, Duque A, Erikson DL, Ewango CEN, Fischer GA, Fletcher C, Foster RB, Giardina CP, Gilbert GS, Gunatilleke N, Gunatilleke S, Hao Z, Hargrove WW, Hart TB Hau, BCH, He F, Hoffman FM, Howe RW, Hubbell SP, Inman-Narahari FM, Jansen PA, Jiang M, Johnson DJ, Kanzaki M, Kassim AR, Kenfack D, Kibet S, Kinnaird MF, Korte L, Kral K, Kumar J, Larson AJ, Li Y, Li X, Liu S, Lum SKY, Lutz JA, Ma K, Maddalena DM, Makana J-R, Malhi Y, Marthews T, Mat Serudin R, McMahon SM, McShea WJ, Memiaghe HR, Mi X, Mizono T, Morecroft M, Myers JA, Novotny V, de Oliveira AA, Ong PS, Orwig DA, Ostertag R, den Ouden J, Parker GG, Phillips RP, Sack L, Sainge MN, Sang W, Sri Ngernyuang K, Sukumar R, Sun, I-F, Sungpalee W, Suresh HS, Tan S, Thomas SC, Thomas DW, Thompson J, Turner BL, Uriarte M, Valencia R, Vallejo MI, Vicentini A, Vrska T, Wang X, Wang X, Weiblen G, Wolf A, Xu H, Yap S, Zimmerman J. 2015. CTFS-ForestGeo: a worldwide network monitoring forests in an era of global change. Global Change Biology, 21, 528-549. pdf Supporting Information
Anderson-Teixeira KJ, Davies SJ, Bennett AC, Gonzalez-Akrea EB, Muller-Landau HC, Wright SJ, Abu Salim K, Almeyda Zambrano AM, Alonso A, Baltzer JL, Basset Y, Bourg NA, Broadbent EN, Brockelman WY Bunyavejchewin S, Burslem DFRP, Butt N, Cao M, Cardenas D, Chuyong GB, Clay K, Cordell S, Dattaraja HS, Deng X, Detto M, Du X, Duque A, Erikson DL, Ewango CEN, Fischer GA, Fletcher C, Foster RB, Giardina CP, Gilbert GS, Gunatilleke N, Gunatilleke S, Hao Z, Hargrove WW, Hart TB Hau, BCH, He F, Hoffman FM, Howe RW, Hubbell SP, Inman-Narahari FM, Jansen PA, Jiang M, Johnson DJ, Kanzaki M, Kassim AR, Kenfack D, Kibet S, Kinnaird MF, Korte L, Kral K, Kumar J, Larson AJ, Li Y, Li X, Liu S, Lum SKY, Lutz JA, Ma K, Maddalena DM, Makana J-R, Malhi Y, Marthews T, Mat Serudin R, McMahon SM, McShea WJ, Memiaghe HR, Mi X, Mizono T, Morecroft M, Myers JA, Novotny V, de Oliveira AA, Ong PS, Orwig DA, Ostertag R, den Ouden J, Parker GG, Phillips RP, Sack L, Sainge MN, Sang W, Sri Ngernyuang K, Sukumar R, Sun, I-F, Sungpalee W, Suresh HS, Tan S, Thomas SC, Thomas DW, Thompson J, Turner BL, Uriarte M, Valencia R, Vallejo MI, Vicentini A, Vrska T, Wang X, Wang X, Weiblen G, Wolf A, Xu H, Yap S, Zimmerman J. 2015. CTFS-ForestGeo: a worldwide network monitoring forests in an era of global change. Global Change Biology, 21, 528-549. pdf Supporting Information
Chu C, Bartlett M, Wang Y, He F, Weiner J, Chave J, Sack L. 2016. Does climate directly influence NPP globally? Global Change Biology 22, 12-24. pdf Supporting Information
Granda E, Scoffoni C, Rubio-Casal AE, Sack L, Valladares F. 2014. Leaf and stem physiological responses to summer and winter extremes of woody species across temperate ecosystems. Oikos 123, 1281-1290. pdf Appendix
 Moles AT, Perkins SE, Laffan SW, Flores-Moreno H, Awasthy M, Tindall ML, Sack L, Pitman A, Kattge J, Aarssen LW, Anand M, Bahn M, and 36 others. 2014. Which is a better predictor of plant traits: temperature or precipitation? Journal of Vegetation Science 25, 1167-1180. pdf
Moles AT, Perkins SE, Laffan SW, Flores-Moreno H, Awasthy M, Tindall ML, Sack L, Pitman A, Kattge J, Aarssen LW, Anand M, Bahn M, and 36 others. 2014. Which is a better predictor of plant traits: temperature or precipitation? Journal of Vegetation Science 25, 1167-1180. pdf
Ostertag R, Inman-Narahari F, Cordell S, Giardina CP, Sack L. 2014. Forest structure in low-diversity tropical forests: a study of Hawaiian wet and dry forests. PLoS ONE 9: e103268. pdf
Poorter H, Jagodziński A, Ruíz-Peinado R, Kuyah S, Luo Y, Oleksyn J, Usoltsev V, Buckley T, Reich PB, Sack L. 2015 How does biomass allocation change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytologist 208, 736-749. pdf Supporting Information
Development of new methods, applications of technologies and improvement of protocols for plant structure and function
 There are >300,000 plant species worldwide and characterizing their structure, function, physiology and ecology is a formidable aspiration. We desperately need means for making excellent, reliable and preferably rapid measurements that will reveal important aspects of species’ biology, or that can be used to parameterize models for plant function and for prediction of their responses to climate change. In the lab we take a strong focus on developing and improving methodology, and when possible developing suggestions toward standardized methodologies in the field (see also Protocols page). Lawren was a co-founder of PrometheusWiki, created to serve as an up to date resource for protocols for plant and environmental sciences.
There are >300,000 plant species worldwide and characterizing their structure, function, physiology and ecology is a formidable aspiration. We desperately need means for making excellent, reliable and preferably rapid measurements that will reveal important aspects of species’ biology, or that can be used to parameterize models for plant function and for prediction of their responses to climate change. In the lab we take a strong focus on developing and improving methodology, and when possible developing suggestions toward standardized methodologies in the field (see also Protocols page). Lawren was a co-founder of PrometheusWiki, created to serve as an up to date resource for protocols for plant and environmental sciences.
Selected publications:
Sack L, Caringella M, Scoffoni C, Mason C, Rawls M, Markesteijn L, Poorter L. 2014. Leaf vein length per unit area is not intrinsically dependent on image magnification: avoiding measurement artifacts for accuracy and precision. Plant Physiology 166, 829-838. pdf Supplemental Data
Bartlett M, Scoffoni C, Ardy R*, Zhang Y, Sun S, Cao K, Sack L. 2012. Rapid determination of comparative drought tolerance traits: using an osmometer to predict turgor loss point. Methods in Ecology and Evolution 3, 880-888. pdf Supporting Information
Sack L, Scoffoni C. 2012. Measurement of leaf hydraulic conductance and stomatal conductance and their responses to irradiance and dehydration using the evaporative flux method (EFM). Journal of Visualized Experiments. pdf Movie
Inman-Narahari F, Giardina C, Ostertag R, Cordell S, Sack L. 2010. Digital data collection in forest dynamics plots. Methods in Ecology and Evolution 1: 274-279. pdf Supporting Information
Sack L, Cornwell WK, Santiago LS, Barbour MM, Choat B, Evans JR, Munns R, Nicotra A. 2010. A unique web resource for physiology, ecology and the environmental sciences: PrometheusWiki. Functional Plant Biology 387: 687-693. pdf PrometheusWiki Website