Our research examines how stressors such as chronic diseases alter the dynamics and plasticity of neurons and neural microcircuits.
We focus on the neurodegenerative motor neuron disease, Amyotrophic Lateral Sclerosis (ALS), commonly known as Lou Gehrig’s disease. ALS is characterized by progressive loss of motor neurons, the final common pathway neurons which drive all skeletal musculature. The diverse causes and progression rates of ALS have hampered its early diagnosis and effective treatment. Through the development of a comprehensive roadmap of disease dynamics beginning at the neonatal stage, we seek to establish early biomarkers and devise ways to delay the onset and progression of neurodegeneration in ALS.
Our ongoing work addresses three key questions:
What makes neurons selectively vulnerable to degeneration?
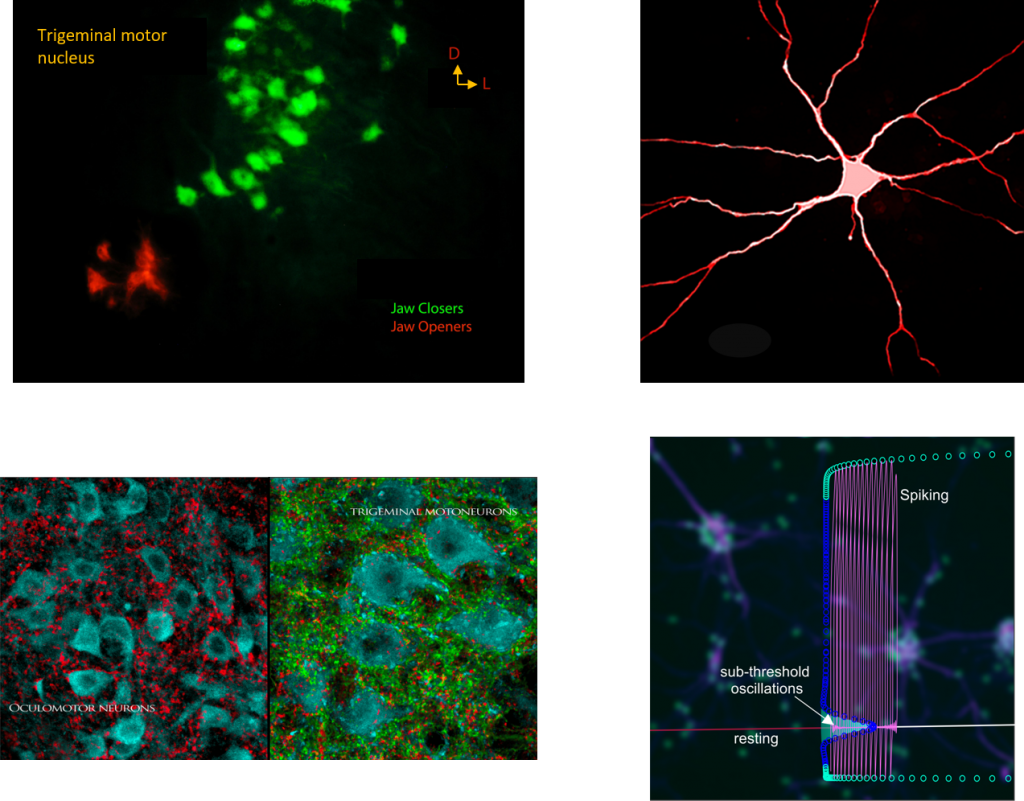
Selective vulnerability is a hallmark of neurodegenerative diseases. However in ALS, not all motor neurons are equally vulnerable. For instance motor neurons controlling eye muscles and those innervating muscle spindles (gamma motor neurons) are resistant to degeneration. Even within vulnerable motor pools, there is preferential death of large motor neurons which are important to produce forceful muscle contractions. Our research explores what properties are different between the vulnerable and resistant motor neurons. Our working hypothesis is that cell-specific markers of disease resistance can offer strategies to delay degeneration of vulnerable neurons.
How are neural circuits reconfigured by the disease?
Normally, neurons regulate their excitability relative to network function – an ability known as homeostatic plasticity. However, neurons often become hyperexcitable during disease development which alters their set point operation. Arguably this change is instigated by a disruption of network homeostasis within so-called vulnerable microcircuits. We are in search of short- and long-range circuit elements including sensory, premotor and cortical neurons as well as astrocytes, microglia and oligodendrocytes, which can contribute to disrupted network homeostasis. Delineating the altered physiology and molecular signatures of these other cells can suggest targets for effective treatment.
What are the dynamic signatures of disease development?
Diseases are elusive and mal-adaptive. To understand such complexity, our unique approach combines mathematical modeling of disease dynamics. Mathematical modeling offers a powerful methodology to integrate discrete experimental findings into integrative computer-based platforms for disease prediction. We develop data-driven models and use them to uncover the promiscuous way in which disease disrupts normal dynamics of ion channels, neurons and networks. We also use these models to test strategies for normalizing neural excitability in real-time. By developing these models, we further seek to delineate clinically relevant physiological parameters and operational regimes to aid disease prediction and design of effective therapeutic strategies.